Spinal Cord Injuries: New Treatments Can Restore the Function of Nerves
Anyone who has ever visited a spinal injury ward in a hospital knows the heartbreak that affects not only the patients but their families. Recovering from spinal nerve damage is a long road through rehabilitation therapy, complicated by the wasting of muscles and the tightening of tendons from disuse.
Until now the fact was that nerves, once damaged, were beyond repair. Therapy following an injury to the spinal nerves was usually devoted to maintaining and strengthening the muscles that were not effected. Rumors of some miracle therapy have spotted the news but they always seemed many years away and stuck in the mire of politics over stem cell research and funding appropriations.
Now it seems we have come to an important impass. Two new breakthroughs stand to make it possible for victims of spinal injury to regain most, if not all, of their nerve functioning.
In the most common type of spinal cord trauma, called "compression" injuries, nerves usually are not severed. Instead they are stretched in such a way that the myelin rips apart exposing the nerve fibers. Once the nerve fibers are exposed they short circuit, much like an electrical wire that has lost its insulation, and the result can be paralysis.
In multiple sclerosis the myelin insulation is also destroyed but not by trauma. For some reason the body's own immune system targets the myelin as a "foreign body" and begins to destroy it. MS is called an "auto-immune" disease and affects more than 350,000 people in the United States and 2 million worldwide.
Riyi Shi, a professor in Purdue University's Department of Basic Medical Sciences, School of Veterinary Medicine, Center for Paralysis Research and Weldon School of Biomedical Engineering has been doing research with this new drug on guinea pigs. He reports that 4-aminopyridine-3-methyl hydroxide has restored function to damaged axons, slender fibers that extend from nerve cells and transmit electrical impulses in the spinal cord.
Here's what they did
The researchers first confirmed previous circumstantial evidence suggesting injury causes the myelin insulation to recede, exposing the channels and impairing signal transmission. Laboratory and imaging techniques revealed the exposed channels in damaged axons.
The researchers then subjected spinal cord tissue to stresses that mimic what happens in a compression injury, which stretches nerves. Then they treated the damaged axons with 4-aminopyridine-3-methyl hydroxide. A common injury of this type in humans is "whiplash" where the neck is violently hyperextended from an automobile accident or a fall.
Spinal cord trauma damages the myelin sheath, exposing "fast potassium channels" that are embedded in the axons and are critical for transmitting nerve impulses. These channels cause interference when they are exposed and interrupt the impulses to muscles, resulting in paralysis. The body cannot, it seems, re-grow myelin on its own. Until now, this kind of nerve injury was considered permanent.
The compound is a derivative of the drug 4-aminopyridine, used primarily as a research tool and also to manage symptoms of multiple sclerosis.
The researchers also discovered that 4-aminopyridine-3-methyl hydroxide is a "potassium channel blocker," using a sophistic laboratory technique called "patch clamp" to measure signal conduction. Findings confirmed that the compound prevents the exposed channels from leaking electrical current and enhances nerve conduction in segments of the damaged spinal cord.
The compound could make it possible to sidestep spinal cord damage by enabling axons to transmit signals as though they were still sheathed in myelin, Shi said.
Nerves transmit signals through a series of rapid electrical pulses, or "action potentials." For proper nerve function, the time gap between pulses must be as brief as possible. However, the medicine currently used for MS (4-aminopyridine) unfortunately has been shown to lengthen the gap, or "refractory period," between pulses. The researchers found that this new drug, 4-aminopyridine-3-methyl hydroxide, restores function without affecting the refractory period. That's great news as the damaged nerves perform more like healthy nerves.
Another key advantage of the new compound is that it's about 10 times more potent than the currently prescribed 4-aminopyridine, meaning lower doses can be used to reduce the likelihood of serious side effects.
But what about severed (cut) spinal nerves?
Here again we have some good news. A new study has found that transplantation of stem cells from the lining of the spinal cord, called ependymal stem cells, reverses paralysis associated with spinal cord injuries in laboratory tests. The findings show that the population of these cells after spinal cord injury was many times greater than comparable cells from healthy animal subjects. The results open a new window on spinal cord regenerative strategies.
In the human body there are many different types of cells, each with a special function and internal structure. A muscle cell is different from a blood cell or a nerve cell. Stem cells have the ability to grow and change into any of these different cells thus repairing tissue.
The two broad types of human stem cells are: embryonic stem cells and adult stem cells that are found in adult tissues. In a developing embryo, stem cells can differentiate into all of the specialized embryonic tissues. In adult organisms, stem cells and progenitor cells act as a repair system for the body, replenishing specialized cells, but also maintain the normal turnover of regenerative organs, such as blood, skin, or intestinal tissues.
What makes stem cells so special is their ability to grown and transform into specialized cells with characteristics consistent with cells of various tissues such as muscles or nerves through cell culture. Highly plastic adult stem cells from a variety of sources, including umbilical cord blood and bone marrow, are routinely used in medical therapies. Embryonic cell lines and autologous embryonic stem cells generated through therapeutic cloning have also been proposed as promising candidates for future therapies.
The transplanted stem cells were found to proliferate after spinal cord injury and were recruited by the specific injured area. When these cells were transplanted into animals with spinal cord injury, they regenerated ten times faster while in the transplant subject than similar cells derived from healthy control animals.
So it appears that the injury to the spinal nerves somehow sends a message to the transplanted stem cells, telling them to actively reproduce more cells to make the repairs. And these repairs are not just associated with the nerve fiber itself -- but also to the oligodendrocytes which form the insulating myelin!
Currently, there are no effective therapies to reverse this disabling condition in humans. However, the presence of these stem cells in the adult human spinal cords suggests that stem cell-associated mechanisms might be exploited to repair human spinal cord injuries.
--Miodrag Stojkovic, co-author of the study.
Early intervention is critical for success
Im the March issue of The Journal of Neuroscience Michael Fehlings, MD, PhD, and his colleagues at the Krembil Neuroscience Center at Toronto Western Research Institute and the University of Toronto identified a critical window during which stem cell transplants may be effective.
Working once again with rodents, the team used cells from the brains of adult mice labeled with a fluorescent marker, enabling them to trace the cells after they were transplanted into rats whose spines had been crushed.
Stem cells transplanted up to two weeks after the initial injury survived thanks to a cocktail of growth factors and immune-suppressing drugs the team developed. More than one-third of the transplanted cells traveled along the spinal cord, were incorporated into damaged tissue, developed into the type of cell destroyed at the injured site, and produced myelin, an insulating layer around nerve fibers that transmits signals from the brain.
An injured spinal cord loses its ability to regenerate myelin-forming cells, leading to paralysis. Fehlings showed that where stem cells restored myelin in the injured spine, rats showed some recovery and walked with more coordination.
One new aspect demonstrated by the study is that "the maximal effect of transplanting these cells is early after injury," says New York University School of Medicine professor Moses Chao, PhD. "The timing of neural stem cell application therefore is critical to successful therapy in the injured spinal cord."
One focus of future research will be to determine the reason why stem cells transplanted weeks or months later fail to function or sometimes even survive.
Comments: |

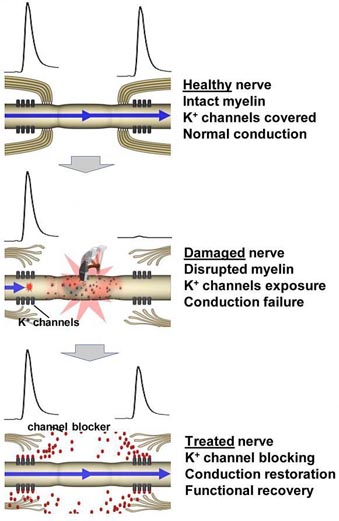 The first breakthrough is a new drug which appears to reverse the effects of nerve damage. As reported in the November issue of Journal of Neurophysiology, researchers have shown how 4-aminopyridine-3-methyl hydroxide can restore the function of nerves damaged in spinal cord injuries by preventing short circuits caused when tiny "potassium channels" in the fibers are exposed. The chemical compound could also be developed as a treatment for multiple sclerosis. Both afflictions have one common problem: the destruction of myelin -- the insulation that surrounds the nerve fibers.
The first breakthrough is a new drug which appears to reverse the effects of nerve damage. As reported in the November issue of Journal of Neurophysiology, researchers have shown how 4-aminopyridine-3-methyl hydroxide can restore the function of nerves damaged in spinal cord injuries by preventing short circuits caused when tiny "potassium channels" in the fibers are exposed. The chemical compound could also be developed as a treatment for multiple sclerosis. Both afflictions have one common problem: the destruction of myelin -- the insulation that surrounds the nerve fibers.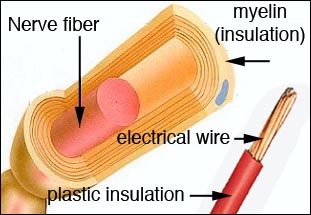 The axon of each nerve fiber are sheathed in a thick insulating lipid (fat) layer, called myelin. This layer insulates each nerve fiber from surrounding nerves and enables the transmission of signals to muscles without short circuiting. It's much like the plastic insulation surrounding electrical wires.
The axon of each nerve fiber are sheathed in a thick insulating lipid (fat) layer, called myelin. This layer insulates each nerve fiber from surrounding nerves and enables the transmission of signals to muscles without short circuiting. It's much like the plastic insulation surrounding electrical wires.  Stem cells are cells found in most, if not all, multi-cellular organisms. They are characterized by the ability to renew themselves through mitotic cell division and to change into a diverse range of specialized cell types.
Stem cells are cells found in most, if not all, multi-cellular organisms. They are characterized by the ability to renew themselves through mitotic cell division and to change into a diverse range of specialized cell types.